HICOF Hector Vision System for Pharmaceutical Labeling Machines
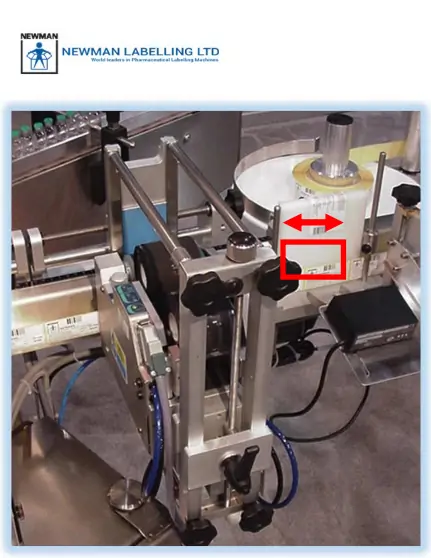
Elevate Your Pharmaceutical Labeling with the HICOF Hector Vision System In the high-stakes world of pharmaceutical manufacturing, precision and compliance are non-negotiable. Consider the HICOF Hector Vision System—an innovation from Switzerland that is designed to provide comprehensive labeling machine vision solutions. Designed with cutting-edge technology, the Hector system protects against costly errors. Whether you’re ensuring […]
HICOF Hector Vision System for Pharmaceutical Labeling Machines Read More »
 Skip to content
Skip to content