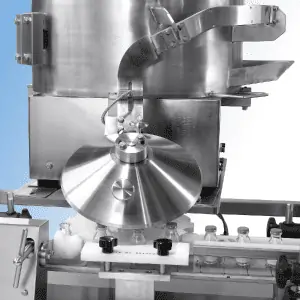
Elevate your packaging security and efficiency with our advanced carton labeling system.
- Processes up to 350 cartons per minute with dual stepper motor-driven label stations
- Handles various carton formats including aeroplane and reverse tuck
- Features built-in sensing technology that rejects improperly labeled products
- Requires zero change parts for different product runs
- Includes validation documentation for regulatory compliance
The stainless steel construction and “positive accept” security system ensure reliable operation in pharmaceutical and other sensitive manufacturing environments. Optional Easisplice modules eliminate downtime during reel changes.
Technical Specifications
Performance Parameters
- Production throughput: Maximum 350 cartons per minute
- Label application: Dual-station configuration for multi-surface labeling
- Compatible packaging: Aeroplane and reverse tuck carton formats
- Label types: Self-adhesive tamper-evident and hologram security labels
Mechanical Design
- Form factor: Space-efficient framework with minimized footprint
- Construction material: Pharmaceutical-grade stainless steel
- Drive system: Precision stepper motors controlling label web advancement
- Carton transport: Top belt conveyance with specialized separation mechanism
- Label application method: Roller-based contact system for vertical carton faces
Quality Control Features
- In-process verification: Real-time label presence detection
- Acceptance protocol: Dual-label verification before downstream release
- Rejection mechanism: Automatic diversion to secured collection area
- System monitoring: Continuous critical component self-diagnosis
- Production tracking: Integrated product and reject counting functionality
Setup & Operation
- Format changes: Tool-less adjustment with calibrated positioning indicators
- Repeatability: All adjustment points feature scaled markings for precise setup
- Validation: Comprehensive documentation package included for regulatory compliance
- Learning curve: Designed for intuitive operation and minimal training requirements
Optional Enhancements
- Continuous operation: Easisplice 470 modules (quantity: 2) enable non-stop production during label reel changeovers
Manufacturing Standards
- Production environment: Designed to Good Manufacturing Practice (GMP) specifications
- Security protocol: “Positive accept” methodology ensures only properly labeled products proceed
This system represents a balance of high-throughput capability and stringent quality control, making it particularly suitable for pharmaceutical, healthcare and other regulated industry applications where package integrity verification is essential.
 Skip to content
Skip to content