The COVID-19 pandemic is changing rapidly and requires different strategies to maintain clinical preventive services, including immunization. The race to develop, produce and distribute the first effective vaccine for the SARS-COV-2 virus has alerted a lot of governments to pre-order huge doses of the vaccine as soon it becomes available and goes into production.
As Covid-19 vaccine trials enter a defining stage, authorities Worldwide have started preliminary discussions on a wide range of issues, from logistics to ethical questions, to set the stage for a smooth supply and effective use of a vaccine when it is ready. With Pfizer and BioNTech announcement that their product is nearing its completion of the Phase 3 trials and claims of urgent production of up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021, we do have some concerns regarding the integrity of the stored vaccines as most of the vaccine administration facilities don’t meet the storage requirements for such a high volume of vials and syringes being stored at a single point in time. Proper vaccine storage and handling practices play a very important role in protecting individuals and communities from vaccine-preventable diseases. Vaccine quality is the shared responsibility of everyone involved, from the time vaccine is manufactured until it is administered.
Our concerns are that developing a successful COVID-19 vaccine is only half the battle. We all know that most vaccines must be kept refrigerated at frozen or near-freezing temperatures to remain stable. That’s a problem when you consider that there isn’t enough refrigerated space in the world to store 7.8 billion vaccine doses. In addition, it’s highly anticipated that many of the vaccines that are being tested right now—or that are in early development stages—are going to need multiple doses, which further complicates the logistics of rapidly delivering vaccines to billions of people. Imagine of having to take 3 revaccinations for the vaccine to be effective.
This means that there is a serious storage concern because at a single point in time we might need to have approximately 24 billion lots of vaccines stored in a freezer or a stability chamber.
Once there is a vaccine for the virus that causes COVID-19, administering it will need to be just as successful as the vaccine itself. If the vaccine needs to be administered in multiple shots —like the HPV vaccine, which requires three—this may significantly reduce how much of the global population is successfully vaccinated.
Addressing the Storage Concerns
The WHO Vaccine Storage Guidelines provide information and advice for vaccine storage management for immunization service providers, from medical practices to large hospitals, clinics and outreach providers.
WHO refers to 5 degrees Celsius (°C) — that is, the point midway between +2°C and +8°C which is the temperature range recommended for vaccine storage. Many vaccines are damaged or destroyed at temperatures outside this range.
With the help of Environmental Specialties‘ Steve Ferguson, we did a comprehensive webinar broadcast where we discussed the proper vaccine storage methodologies. Due to the current trends, we are now offering a rerun of the same talk which was recorded in 2017.
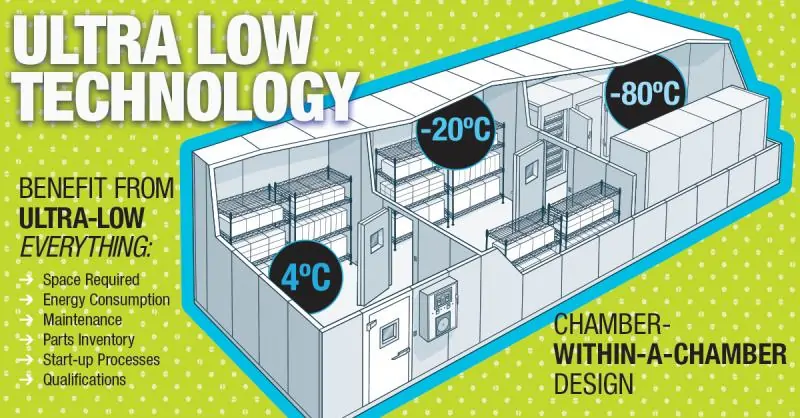
Environmental Specialties (ES) designs and manufactures a diverse product line of controlled environmental chambers/rooms throughout a wide variety of industries.
In the pharmaceutical market, ES’ focuses on stability rooms meeting and exceeding the ranges and uniformities of the ICH has allowed us to become the recognized leader in chamber design, construction, and stringent performance qualification in support of our clients’ validation.
For the growing demand for ultra low temperature storage for vaccines, ES manufactures the Low Temp (LT) chamber series. This product series includes the Low Temp Chest (LTC), Low Temp Upright (LTU), and the popular Low Temp Walk-in (LTW).
The products provide from approximately 80 cubic feet to over 1000 cubic feet of ultra low -70 to -80 C freezer storage area, and are typically used to support biorepository needs for ultra cold temp storage freezer vaults or cold storage vaults.
All products are custom designed to a specific size, temperature, and electrical control specifications. Typical storage includes small vials, plates, bulk drums, and canisters of various biological samples and vaccine products.
Maximum capacity, multi-temperature and storage flexibility and product protection are essential to design elements available in the most unique cascade freezers on the market today.
This is actually a new solution to the antiquated ‘freezer farm’ approach which uses individual freezers with single, common refrigeration systems to store large volumes of products. This is finally, a solution better than “just adding one more freezer”.
Our final advice will be to also check out the webinar we did with Dan Gresens of Environmental Specialties where he explained the concepts for Site Considerations and Preparations for Environmental Chambers.
If you want to have a further conversation on how we can help out with your vaccine storage needs, please get in touch with us via 800-829-5741.
 Skip to content
Skip to content